

GD — Society for Dermopharmacy
 |
Issue 1 (2005) |
Authors' Article
Survey:
Transparency for dermatic bases
R. Eifler-Bollen und H. Reimann, Eschborn
There is still no complete transparency at the suitability and quality of dermatic bases according to the pharmaceutical law. This is the reason for the NRF to establish a review of the brand principles for which a medical prescription in accordance with formulation is documented.
The Pharmazeutische Laboratorium of the Neue Rezeptur-Formularium (Pharmaceutical Laboratory of the New Prescription Formulary) (NRF) conducted a survey in cooperation with the Gesellschaft für Dermopharmazie (GD) in autumn 2004 aiming at the qualification of bases preponderantly used in cosmetics. Inducement of this survey was the second consensus conference of the guideline "Dermatological Prescription" of the GD [1]. Experts from administration, ministerial surveillance, dermatological practice, pharmacies, universities, clinics, specialized and occupational associations and industry took part in the consultancy.
Unanimously the participants called for complete transparency in the formal suitability and quality of dermatic bases if they are applied for prescription drugs. For physicians are often not aware of the fact that pharmacists are obliged to substantiate the required pharmaceutical quality of the starting substances [1-3] according to Pharmaceuticals Law (§ 55 AMG) and Pharmacist Operating Rules (§§ 6 and 11 ApBetrO) also for medical prescriptions. In case this is not feasible for dermatic bases the manufacturing of the drug is not permissible.
Target of the survey was therefore a review of more than 50 brand bases for which a medical prescription according to formulation is documented at the NRF. 20 producers were asked for information concerning their pharmaceutical quality and suitability for the manufacturing of prescription drugs. 17 producers respectively vendors rendered this self-disclosure.
Pharmaceuticals
and/or Cosmetics
The least problems are expected for the use of officinal bases from the pharmacopoeia and Deutsche Arzneimittel-Codex (DAC) (German Drug codex) accompanied by valid test certificate [1, 3, 4] as well as medicinal approved dermatic bases. For ready-to-use drugs an admittance dossier is available and the drug production is compliant to the German Pharmaceutical Act. Some dermatics are for example admitted for the interval therapy.
In contrast initially it seems hardly imaginable that a cosmetic may be consistent with the stipulations of the operating regulations of the pharmacies as prescription ingredient. If, however a brand base is not only produced according to the standards of the 'Cosmetics-GMP' of the cosmetics regulation but also pursuant to the stipulations of the pharmaceutical law, the afore established pharmaceutical quality is naturally not lost by its marketing as cosmetic. A base of the kind can therefore equally be processed and formulated as a base exclusively intended for the drug production.
Detrimental for the determination of the required quality of a product intended for a prescription ingredient is the fact that for the pharmacist and physician this legally formal background is not self-explanatory as the products are only seldom accompanied by the test certificate according to § 6 Abs. 3 ApBetrO. In general, it is expected that the pharmacist also adheres to the principle of merely applying "known" starting materials in the medicinal sciences in the area of non-compulsory approval drugs in prescription and defecture. Not known by authorities for dermatics are however numerous preservatives, colorants, UV-filters or other substances contained in cosmetic products which are considered to be critical [5].
A first step
Responses submitted by producers - respectively sales companies can be gathered from the table. The latter however does not raise claim to be complete. Three producers respectively entrepreneurs did not answer to the survey and have therefore not been considered. The alphabetic indication of the companies obviates an accentuation of individual companies. Also the persistently descriptive character and the missing of an objective and professional assessment by the authors does not allow inference as concerns quality and suitability from sequence or form of representation.
Responses by producers to individual questions respectively sales companies have been complemented by remarks and cited in the form of footnotes a through I. These allow the presumption that there may be a certain optimization requirement in details. Exemplary this shows for question 2 concerning the exclusive use of pre-tested pharmacological ingredients for production. The monograph in the pharmacopoeia is here a definite basis for decision. There is however a certain scope for discussion and discretion regarding the test standards at ingredients not at all or insufficiently described in the pharmacopoeia or DAC.
The toxicological assessment for ingredients which are marketed in cosmetic products is of similar problematic nature, the harmlessness of which has not been subject to an official licensing procedure in view of the pharmaceutical law. Who is to decide here that for example the preservatives polihexanide or methyl chloroisothiazolinone used in cosmetics are allowed for use in prescription drugs? The question actually posed in number 8 for these substances has often been misunderstood and has therefore not been considered in the assessment.
Altogether, the compiled
producer information represents a multitude of relevant information available
at one glance to the physician and pharmacist and is a first important
step towards increased transparency and safety in the prescription routine.
Literature
- Leitlinie Dermatologische Rezepturen, GD – Gesellschaft für
Dermopharmazie e.V., Fassung 1.4.2003,
http://www.gd-online.de/german/fgruppen/magistral/leitlinienmagistral.htm - Isermann, D., Die Magistralrezeptur, Kosmetische Medizin 5 (2001) 248–250.
- Bundesapothekerkammer, Leitlinien zur Qualitätssicherung. Herstellung
und Prüfung der nicht sterilen Rezeptur- und Defekturarzneimittel,
Revision 6.5.2003,
www.abda.de/ABDA/download/Qualitaetssicherung/Nicht_sterile_Rez.pdf. - Ali, S. L., Ihrig, M., Ausgangsstoffe und valide Analysenzertifikate, Pharm. Ztg. 146 (2001) 2630–2631.
- AMK-Stellungnahme Bedenkliche Rezepturarzneimittel, Pharm. Ztg. 149 (2004) 1800–1801.
Pharmazeutisches Laboratorium des NRF, Carl-Mannich-Straße 20, 65760 Eschborn, E-Mail: nrf@govi.de
Questionnaire for the assessment
of pharmaceutical quality of
dermatics brand bases for the
preparation of formulations
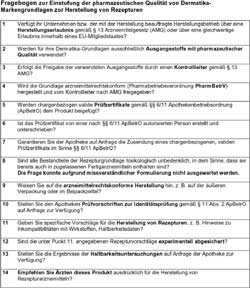
Enlarged version
Table: Survey results regarding
prescription bases
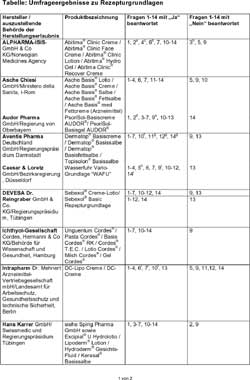
Enlarged
version
a) provided that raw materials in Ph. Euro. are described.
b) involvement of qualified person (QP) only for out-of-specification
results
c) Preparation according to GMP, release is effected according to chapter
3.
d) if available.
e) certificate of analysis.
f) In the individual case against declaration of confidentiality.
g) Until 1993 prescription proposals reviewed by galenics were made available.
Explicit operational instructions have not been issued.
h) are retrievable in the internet at www.caelo.de
i) on the certificate of analysis.
j) formulation is based on DAC-Monograph.
k) with exception of Excipial cream, all products of the company Spirig
indicated in the list above are authorized in Switzerland as medicament.
l) Intrapharm Dr. Mehnert schedules the securing of information regarding
questions 6, 7 and 10 for the year 2005 (objective 2nd quarter).