GD — Society for Dermopharmacy
 |
Issue 1 (2003) |
Authors' Articles
Jean Krutmann*
Targets for Repair-Products against Photoaging
*Prof. Dr. med. Jean Krutmann presented this paper at a symposium of the GD Society for Dermopharmacy in Duesseldorf, October 17th 2001.
Introduction
Ultraviolet (UV) radiation is well known to exert a variety of deleterious effects on human skin. Chronic and possibly also acute exposure to UV radiation increases the risk of developing skin cancer including basal-cell and squamous-cell carcinoma as well as malignant melanoma. UV radiation causes skin aging which is characterized by generalized wrinkling, dry and thin appearance and seborrheic keratoses (Figure 1).
Extended life span, more spare time and excessive exposure to UV radiation from natural sunlight or tanning devices, especially in the western population, have resulted in an ever increasing demand to protect the human skin against these detrimental effects. As a consequence, technologically highly advanced sunscreens have been developed within recent years which contain physical and chemical UV filters. These sunscreen preparations have proven to be quite effective, in particular when used for the prevention of the erythema (sunburn) reaction of human skin.
 |
There
is an ongoing debate whether UV filters also protect against other harmful
effects besides the sunburn reaction. From this discussion, it has been
learned that the answer to this question is very complex. There is increasing
evidence that sunscreens which protect against a sunburn also protect
human skin against other harmful effects, but this protection may be superior,
equal or less effective to that observed for erythema prevention. Also,
for some of the biological endpoints which are relevant for photocarcinogenesis
or photoaging, no in vivo assays exist which would allow to test the efficacy
of a given sunscreen. In summary, there is currently no doubt that the
regular use of UV-filter-containing sunscreens should be recommended to
the public as the best available photoprotection (besides staying out
of the sun and/or wearing clothes), but at the same time it is important
to realize that sunscreens are not yet perfect. As a consequence, a number
of active ingredients have been developed more recently which are being
combined with or used in addition to UV filters to enhance the degree
of protection which can be achieved with sunscreens only. In addition,
they have fostered the development of novel test models which allow for
the first time to prove or disprove the efficacy of UV filters and active
agents for specific biological endpoints which are thought to be of greater
relevance to human health than the sunburn reaction.
This review will summarize studies which have been performed to a major
extent in the authors´s laboratory and which illustrate this development
with two examples: (1) the use of topically applied DNA repair enzymes
to prevent UVB-radiation-induced skin damage and (2) the development of
a novel, combined in-vitro/in vivo assay to measure the efficacy of UV
filters, antioxidants or selected active agents to protect human skin
against UVA-radiation-induced photoaging.
UVB
Photoprotection with Topically
Applied DNA Repair Enzymes
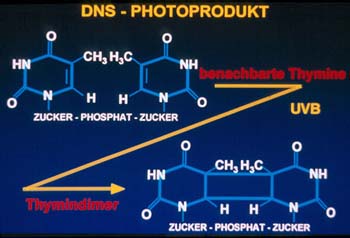
The induction of photoproducts within the DNA of epidermal cells is detrimental
to human health. Among the DNA lesions induced by UVB radiation, cyclobutane
pyrimidine dimers predominate (Figure 2).
Dimer formation is thought to be crucial for the initiation of skin cancer,
because it was found to be closely linked to the generation of mutations
in tumor suppressor genes expressed in UV-induced skin cancer. There is
also growing evidence that dimers contribute to photocarcinogenesis by the
suppression of the skin´s immune system, allowing transformed cells
to grow unimpeded. Strategies directed at the removal of dimers from UVB-irradiated
human skin are thus of paramount concern for photoprotection.
Conventional photoprotection by sunscreens, however, is exclusively prophylactic
in nature and of no value once DNA damage has occurred. In collaboration
with AGI Dermatics, Freeport, N. Y., USA, we have therefore recently assessed
whether it is possible to repair UVB-radiation-induced DNA damage by topical
application of a DNA repair enzyme. In these studies, the DNA repair enzyme
photolyase was used which specifically converts cyclobutane dimers into
their original DNA structure after exposure to photoreactivating light.
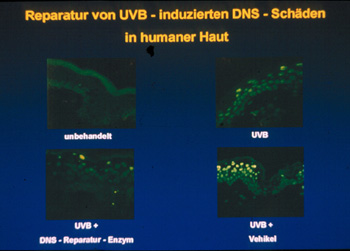
Topical application of photolyase-containing liposomes to UVB-irradiated
skin and subsequent exposure to photoreactivating light decreased the number
of UVB-radiation-induced dimers by 40 - 45 % (Figure 3).
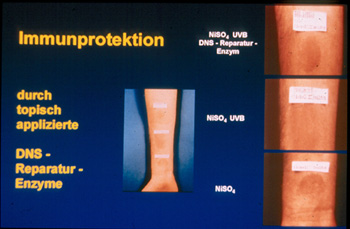
Partial repair was of functional relevance because it provided immunprotection
to UVB-irradiated human skin. In individuals hypersensitive to nickel sulfate,
UVB radiation suppressed the elicitation phase of the hypersensitivity reaction
to nickel sulfate, and this immunosuppressive effect was completely prevented
by partial repair of UVB-radiation-induced dimers which could be achieved
by the topical application of photolyase-containing liposomes on UVB-irradiated
human skin and subsequent exposure to photoreactivating light (Figure
4).
In summary, these studies indicate that topical application of exogenous
DNA repair enzymes to human skin is an approach that is highly effective
in protecting human skin from the deleterious effects that result from the
presence of UVB-radiation-induced pyrimidine dimers. The exogenous application
of DNA repair enzymes differs from conventional photoprotection by its ability
to remove damage that has already occurred. This approach could thus be
ideally combined as an aftersun strategy with conventional sunscreens to
provide photoprotection and repair at the same time. In fact, aftersun preparations
as well as sunscreen formulations containing liposomes filled with biologically
active photolyase have now been made available to the public by the cosmetic
industry and the efficacy of these preparations has been proven both in-vitro
and in-vivo. Ongoing studies are also directed at assessing the efficacy
of this novel photoprotective approach to prevent UVB-radiation-induced
photoaging.
Mitochondrial DNA Mutations
An
in vitro/in vivo Biomarker to Evaluate Protection against Photoaging
UVB and UVA radiation differ in their photophysical properties and penetrate
into human skin to different extents. Accordingly, the shorter-wavelength
UVB radiation is mostly absorbed in the epidermis and predominantly affects
epidermal keratinocytes and Langerhans cells, while the longer-wavelength
UVA radiation penetrates more deeply and can interact with both epidermal
cells and dermal fibroblasts.
Recent studies from our
laboratory indicate that a direct interaction of UVA radiation with the
latter cell population is of enormous relevance for photoaging of human
skin. This newly recognized pathway through which UVA radiation triggers
photoaging of human skin is initiated in dermal fibroblasts by alterations
at the level of mitochondrial (mt) DNA.
Evidence for a critical role of mtDNA mutations in photoaging of human skin
originates from studies which demonstrate that chronically sun-exposed skin
showing clinical signs of photoaging has a higher mutation frequency of
the mtDNA than sun protected skin (Figure 5).
Even more importantly, the capacity of solar UVA radiation to cause the
formation of mtDNA mutations has been proven in our laboratory through the
development of a combined in-vitro and in-vivo model which allows the generation
of mtDNA mutations in human dermal fibroblasts through repetitive, low-dose
UVA irradiation of cultured human dermal fibroblasts or previously sun-protected
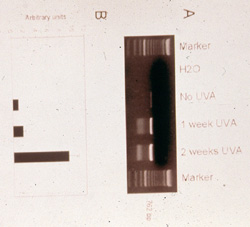
human skin, respectively. Accordingly, normal human fibroblasts, when repetitively
exposed for 3 weeks to sublethal, nonapoptogenic doses of UVA radiation
exhibit a time- and dose-dependent increase in the most frequent mtDNA mutation,
the so called common deletion (Figure 6).
In a very similar manner, repetitive irradiation of normal human buttock
skin also led to the induction of the common deletion. Most interestingly,
in human skin the common deletion could still be detected up to 18 months
after cessation of the irradiation regimen, strongly indicating that the
common deletion serves as memory function in human skin for previously inflicted
actinic damage.
By employing this combined in vitro/in vivo model for photoaging of human
skin, it has been possible to assess the efficacy of sunscreens, antioxidants
and active agents to provide protection against photoaging. Accordingly,
broad-spectrum sunscreens filtering in both the UVB and UVA range were found
to prevent the generation of the common deletion in vivo in human skin.
Moreover, studies on the photobiological mechanisms underlying the UVA-radiation-induced
generation of the common deletion revealed a crucial role for the generation
of ROS and, in particular, for singlet oxygen in this process, and several
antioxidants were subsequently shown to be capable of preventing the UVA-radiation-induced
generation of the common deletion. It is anticipated that the in vitro/in
vivo model described above will not only be extremely useful to prove or
disprove cosmetic claims concerning protection of human skin against photoaging,
as has already been demonstrated, but will also allow to search for new
active substances which can help to prevent or, even more fascinatingly,
repair mtDNA mutations in chronically UVA-irradiated human skin. Improving
the repair of already existing damage caused by both UVB and UVA radiation
would complete a strategy to decrease the detrimental effects of sun exposure.
Author
Universitäts-Professor Dr. med. Jean Krutmann

Clinical and Experimental Photodermatology, Dermatological Clinic, Heinrich-Heine-Universitaet
Duesseldorf,
and
Institute for Environmental Medical Research gGmbH at Heinrich-Heine-Universitaet
Duesseldorf
top