GD — Society for Dermopharmacy
 |
Issue 1 (2005) |
Authors' Article
Realistic expectations to UVA-protection by sun protection agents with high or very high sun protection factor
A. Posselt, R. Daniels
Introduction
Excessive sun exposure impairs the skin. The part of the sunlight relevant for this damage is located in the ultraviolet area (UV) (illustration 1). Whoever cannot or does not want to avoid intensive sun exposure resorts frequently to sun protection agents. Thereby it is understood by experts but also by most laymen, that UVB radiation is able to cause at least sunburn. Meanwhile it is self-evident for almost everyone to prevent the development of a sun erythema by applying products with sufficient light protection factor (sun protection factor LSF, LF or SPF). The increasing sanitary awareness is reflected by consumers' behaviour of deciding more and more frequently in favour of products with high (SPF 15, 20, 25) and very high (SPF 30 and above) light protection factor.
 Illustration 1: Spectral partition of different wave-lengths sectors in the light in ultra-violet, visible and infrared radiation |
The product declaration in view of UVB protection is effected consistently based on the light protection factor (LSF, LF or SPF) which is determined according to an in-vivo method standardized by COLIPA (1). The consumer is familiar with the declaration and pays regard to it when selecting a product.
The situation is to a much lesser degree stringent regarding UVA radiation and UVA protection, although intense research activities of the last years were equally able to definitely prove a damaging effect of the long-wave UV rays. Primarily the focus is set on extrinsic skin ageing (2). The light aging of skin (photo-aging) leaves visible traces - also visible for the consumer. Moreover, UVA-radiation has been identified as triggering factor for light dermatoses. Alike, evidence for the damage of the cell nuclei of skin cells has been proven (3).
Nevertheless, to date the degree of UVA protection has been of minor importance in the product selection. The orientation by the consumer is rendered additionally difficult by the fact that the determination of the degree of the UVA protection and its pertinent declaration is not standardized. Most producers adhere to the stipulations by the Australian standard (4) when determining the UVA protection. Its requirements are met as soon as a sun protection agent reduces the transmission in the area of 320 - 360 nm by at least 90 %. The Australian standard does not provide a further differentiation above this threshold level. This is to ensure an adequate UVA protection when adhering to this standard at products with low SPF, with increasing SPF however, the UVA protection may stagnate at a constant level without infringing the standard; the UVA protection thus does not necessarily increase with the UVB protection. This leads to the fact that until reaching of the erythema threshold with increasing SPF of the sun protection products used, an increasing UVA dose affects the skin (illustration 2).
Enlarged version
This dissatisfactory situation lead in the past to a development of tools in national and international dermatological working groups parallel to the development of product concepts allowing for a higher UVA protection rendering possible a differentiated measurement and labelling of the UVA protection. A first step in direction of an international harmonized way of proceedings, a new German industry standard (DIN 67502) (5) has been established since February 2005. The specific feature of the method described in this standard is that the UV protection is first of all determined in vitro and subsequently a relationship is established to the in vivo light protection factor of the test product and the measuring value obtained.
According to the principle
of DIN 67502, the UVA protection performance of products with SPF 10-12
has already been established (6, 7) in the last years. In this context
it has been ascertained that products with the highest UVA protection
performance are offered by producers who have been engaged in research
and development in the issue sun protection for years. Against it discount
products show on average only a relatively low UVA protection.
The investigation available aims at assessing the UVA protection performance of sun protection agents currently available in the market. The study includes this time in essence sun protection products with light protection factors (SPF) between 20 and 30 as well as sun protection products for children with a SPF between 25 and 30. By applying DIN 67502 it is to analyze whether the increased UVB protection is connected to an adequately high UVA protection.
A particular focus
is set on sun protection products for children as for these a significant
performance increase in view of the light protection factors could be
observed in the last years. This aligns with the recommendation to use
sun protection agents with higher light protection factors for children
as many of the natural protection mechanisms of the skin are only developed
between the second year of age and the puberty. Unrestrictedly recommendable
products are to prevent the particularly fragile infantile skin from sunburn
by particularly high light protection factors and by adapted UVA protection
performance at the same time from long-term light related damage.
Assays investigated
In total 31 sun protection products had been purchased in chemist's shops, in grocer's shops and pharmacies for the tests. Subject to testing were body lotions, facial creams and sprays with varying UV filter systems and SPF values between 15 and 40; thereunder 19 standard and 12 sun protection for children. On most products the UVA-/UVB wideband protection according to the Australian standard AS 2604 was indicated. Only in rare cases the feature of a particularly well-balanced or equilibrated relationship of UVA and UVB protection - without reference to the Australian standard - has been claimed.
Detailed information regarding the SPF, product category as well as filter properties are to be found in table 1 and table 2.
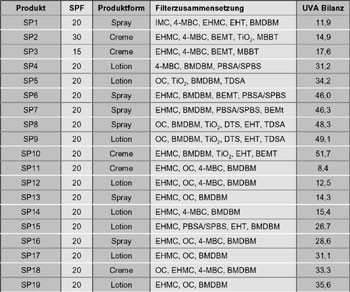 Table 1: Overview of the standard sun protection products included in the test |
Abbreviations:
BEMT= Bis-Ethylhexyloxyphenol
Methoxyphenol Methoxyphenyl Triazine
BMDBM= Butyl Methoxydibenzoylmethane
DBT = Dietyhlhexyl Butamido Triazone
DTS = Drometrizole Trisiloxane
EHMC = Ethylhexyl Methoxycinnamate
EHT = Ethylhexyl Triazone
HD = Hydrodispersion
IMC = Isoamyl p-Methoxycinnamate
MBC = Methylbenzylidene Camphor
OC = Octocrylene
SPBS = Sodium Phenylbenzimidazole Sulfonate
TiO2 = Titanium Dioxide
TDSA = Terephthalidene Dicamphor Sulfonic Acid
ZnO = Zinc Oxide
 Table 2: Overview of the sun protection products for children included in the test |
Abbreviations:
BEMT= Bis-Ethylhexyloxyphenol
Methoxyphenol Methoxyphenyl Triazine
BMDBM= Butyl Methoxydibenzoylmethane
DBT = Dietyhlhexyl Butamido Triazone
DTS = Drometrizole Trisiloxane
EHMC = Ethylhexyl Methoxycinnamate
EHT = Ethylhexyl Triazone
HD = Hydrodispersion
IMC = Isoamyl p-Methoxycinnamate
MBC = Methylbenzylidene Camphor
OC = Octocrylene
SPBS = Sodium Phenylbenzimidazole Sulfonate
TiO2 = Titanium Dioxide
TDSA = Terephthalidene Dicamphor Sulfonic Acid
ZnO = Zinc Oxide
Determination of UVA-Balance
The UVA protection performance has been calculated as UVA balance as described in DIN 67502.
The determination
of the UVA balance is based on the designation of the spectral transmission
level τ(λ)
of a sample in the wave-length area 290 nm ≤ ? ≤ 400 nm.
For this aim a sun protection agent in a quantity of 0.75 mg/cm2 is evenly
spread one-sided on an abraded Plexiglas (PMMA) plate and measured by
means of a suited spectral photometer (LOT-ORIEL-INSTASPECII component
appliance) which allows through the array of the sample nearby to an area
integration unit (e. g. Ulbricht bowl, diffuser) to also include the diffuse
part of the transmitted radiation. 3 plates are prepared for each product
on which 4 individual measurements are performed. If the absorption exceeds
the value of 2, the procedure is repeated at correspondingly reduced application
volume (8).
By means of the spectral transmission degree of a sample in the UV-area (290 nm up to 400 nm) first of all the in vitro sun protection factor SFin vitro, er is calculated (according to DIN 5031-10). In case of discrepancy to the in vivo sun protection factor SFin vivo, er indicated on the package, the extinction curve is adapted via a constant. The resulting correction factor is drawn on for the continuative calculation of the in vitro PPD-protection factor SFin vitro, PPD, i. e. a UVA-relevant biological endpoint.
Thereby the PPD (persistent pigment darkening)-performance spectrum (9) and spectral radiation strength Eλ(λ) of a reference UVA radiation source is used (see annex A). This corresponds to the requirements for radiation sources for the determination of the in vivo PPD protection factor SFin vivo, PPD according to the Japanese test procedure (10).
The UVA balance is
calculated on basis of the in vivo sun protection factor SFin vivo,
er and the SFin vitro, PPD which assesses the relationship
of the UVA protection to the accounted sun protection factor SFin vivo,
er. The UVA balance is defined by:

Result and discussion
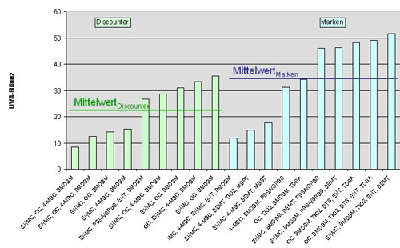
Also for the analysis of products with higher light protection factor the new DIN method for the determination of the UVA protection performance can be applied without limitation and without experiencing difficulties. The individual results can be found in table 1 and table 2. The UVA balance values established vary in the sector of 6.1 to 51.7. This means that differences are even more pronounced for products with higher SPF than for products with SPF 10 to 12 which were tested in the preceding years (6, 7). This large range was nevertheless predictable: for products with higher protection a marked difference is nearly unavoidable depending on whether a product comes just up to the Australian standard or shows an optimally balanced UVA and UVB protection.
Owing to the experience of the previous years, the UVA balance values have been considered differentiated according to the origin of the products, i. e. whether they are from researching producers or from discount stores. In the group of standard products sun creams, lotions and sprays by researching producers performed on average better by 54 percent compared to products from discount stores (illustration 3).
The optimal UVA balance
value of 51.7 obtained a brand. In contrast the best sun protection agent
in the group of the products from discount stores did not exceed the UVA
balance value of 35.6.
Enlarged version
The difference in quality of tested sun protection products for children was even more pronounced in view of the UVA protection performance (illustration 4). In this group all products by research-active producers excel by higher UVA balance values in contrast to the products from discount stores. The best brand protects more than eight times better with a UVA balance value of 51.7 from UVA rays compared to the discount product with the poorest UVA balance value of only 6.1.
On overage the protection of sunscreen agents for children by brand producers is twice as effective as comparable products from discount stores: children products of brand producers obtained a mean value for UVA protection of 41.3. The mean value for children products available at discount stores against it only reached 19.7.
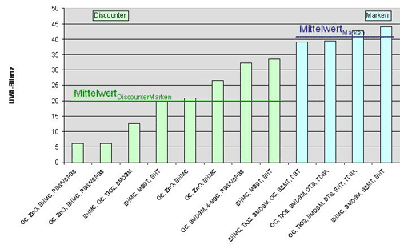 Illustration 4: UVA-balance of sun protection products for children tested divided by brand and discount store products as well as calculated mean values for the respective groups |
Conclusion
On the basis of the substantiated findings about the consequences of UV radiation, experts claim unanimously sun protection products comprehensively shielding both in the UVB and UVA area (11, 12).
Products available in the market come up to these requirements only to some extent. Test results show a distinctive differentiation in the UVA protection performance of the products tested. Exclusively products of research-active producers fulfil the demand relating to a consistent UVB and UVA protection which is to exceed significantly the minimal requirements of the Australian standard for products with high SPF in the UVA area. In contrast, discount products show clear deficits in this protection aspect although this cannot be concluded from the declaration usual at the present time. In particular, the discount products exhibit deficits in the UVA balance values for children products. The latter seems to be particularly fatal in consideration of the fact that parents have the intention to optimally protect their children.
This is why the pressing
wish is expressed that the new option given by the DIN 67502 for the assessment
of the UVA protection will be quickly implemented into the declaration
and test criteria of consumer organizations. Only thus it can be assumed
that the situation will swiftly change for the benefit of the consumer.
Literature
(1) COLIPA Sun Protection Factor (SPF) Test Method, 1994.
(2) Krutmann, J., Vorzeitige Hautalterung durch ultraviolette Strahlung und andere Umweltnoxen. Hautarzt (2003) 54, 809-817. (premature skin aging by ultra-violet radiation and other environmental noxae)
(4) Australian Standard AS 2604 (1993).
(5) Deutsche Industrienorm 67502. Charakterisierung der UVA-Schutzwirkung von dermalen Sonnenschutzmitteln durch Transmissionsmessungen unter Berücksichtigung des Lichtschutzfaktors. (2005). (German Industry Standard 67502. Characterization of UVA protection effect of dermal sun protection agents by transmission measurements under consideration of the light protection factor).
(6) Träger M.,
Daniels R., Differenzierung der UVA Schutzleistung von Sonnenschutzprodukten.
DermoTopics (2003) (Differentiation of the UVA protection performance
of sun protection products)
http://www.dermotopics.de/german/ausgabe_1_03_d
/sonnenschutzprodukte2003.htm
(7) Posselt A., Daniels
R., UVA Schutzleistung von Sonnenschutzprodukten: Hat sich der Markt verändert?
DermoTopics (2004) (UVA protection performance of sunscreen products:
Have there been changes in the market?)
http://www.dermotopics.de/german/ausgabe1_04_d
/UVAsonnenschutz.htm
(8) Klette, E., Wendel, V., Wittern, K.P., Gers-Barlag; H., A quick, practical test procedure to evaluate the performance of instruments used for in vitro UV protection measurements. Int. J. Cos. Sci. 24 (2002) 323-329.
(9) Japan Cosmetic Industry Association (JCIA). Measurement standard for UVA protection efficacy. Jan. 1, 1996.
(10) Chardon, A., Moyal, D., Hourseau, C., Persistent pigment darkening response as a method for evaluation of ultraviolet A protection assay. In: Sunscreens Development and Regulatory Aspects. 2nd ed. (Lowe, N.J., Shaath, N.A., Pathak, M.A. eds) Marcel Dekker New York (1997) pp 559 - 582
(11) Mitteilung des Bundesinstituts für Risikobewertung Stellungnahme des BfR vom 6. August 2003: UV-Filtersubstanzen in Sonnenschutzmitteln. (2003). (Memorandum of the Federal Institut for Risk Assessment Statement of the BfR of 6 August 2003: UV filter substances in Sun protection Agents)
(12) GD Gesellschaft
für Dermopharmazie, Leitlinie dermokosmetischer Sonnenschutz. 2003;
www.gd-online.de (Guideline of dermocosmetic sun protection)
Author's address
Prof. Dr. Rolf Daniels, Lehrstuhl für Pharmazeutische Technologie,
Pharmazeutisches Institut der Eberhard Karls Universität Tübingen, (chair for pharmaceutical technology, Pharmaceutical Institute of Eberhard Karls University Tübingen)
Auf der Morgenstelle 8,
72076 Tübingen
e-mail: rolf.daniels@uni-tuebingen.de
The author
Prof. Dr. Rolf Daniels did his PhD in Pharmaceutical Technology. Before returning to university he had worked for 2 years in the pharmaceutical research department of a major pharmaceutical company. 1995 he was appointed to a professorship for pharmaceutical technology at the Institute for Pharmaceutical Technology at the Technical University Brunswick. Since April 2005 he holds the chair for Pharmaceutical Technology at Tübingen University. His major fields of research comprise surfactant-free emulsion systems, stability assessment of demi-solid systems and the formulation of biotechnologically extracted active agents. Since 1997 he is head of the department Dermocosmetics of the Gesellschaft für Dermopharmazie (GD).
top