GD — Society for Dermopharmacy
 |
Issue 1 (2003) |
Authors'
Article
Horst
Spielmann
New
Methods for Animal-Experiment-Free Tolerability Tests of Cosmetics
Manuscript following the lecture at 7th Annual Meeting of the GD Gesellschaft
für Dermopharmazie e.V. in Bonn on 2 April 2003
Summary: The tolerability test of cosmetics in animal experiments is subject
to severe criticism in the public in Germany and all EU member states.
This criticism, which emanated from animal protection associations, to
begin with has been taken up in the meantime by consumers, authorities
and governments of the EU member states. After several years' discussion,
the 7th amendment of the EU Cosmetics Directive 76/768/EEC (EU Commission
1976), proposed by the EU Parliament, was accepted (EU directive 2003/15/EG)
on 27 February 2003 by the Council of Ministers of the EU member states.
This amendment designates a gradual restriction of animal experiments
for cosmetics and a ban in 10 years. The reason for this gradual way of
proceedings is the fact that at the time being not all animal experiments
provided for testing of cosmetics can be replaced by animal-experiment-free
methods. At the same time considerable progress has been achieved in the
development, validation and international regulatory approval of novel,
animal-experiment-free test methods in the last 10 years so that the replacement
of animal experiments in the tolerability testing of cosmetics will be
feasible in the near future. Therefore, the progress attained is introduced
which is based on mutual efforts of scientists in laboratories of the
cosmetics industry and other research institutes in the development of
alternative methods for animal experiments.
Legal Stipulations for
Safety-toxicological Tests of
Cosmetics in the EU
It has to be considered for the implementation of the EU Cosmetics Directive 97/18/EG that on the one hand there are substances which are to be exclusively safety-toxicologically tested according to the stipulations of the EU Cosmetics Directive and contrariwise, there are substances which are applied as ingredients in cosmetic products and toxicologically tested as industrial chemicals as they are primarily not employed in cosmetics. The latter substances undergo very thorough testing according to the chemical law pursuant to sanitary and environmental aspects. It is moreover important that cosmetical ready-to-use products may no longer be tested in the animal experiment since the amendment of the Animal Welfare Act in Germany in 1987. This legal stipulation is in the meantime adhered to by all EU member states owing to a self-obligation of the cosmetics industry since the year 2000, because considerable progress has been achieved in the development of animal-experiment-free test methods and at the same time no drawbacks have become known for the customer so far. This promising development confirm both the European association of the manufacturers of cosmetics COLIPA as well as the body of experts of the EU commission for the sanitary assessment of cosmetics SSCNF, the Scientific Committee on Cosmetic Products, and Non-Food Products. Remarkable in this context is that from a scientific, economical and ethical view, encouraging progress has been achieved by a voluntary commitment by the manufacturer of cosmetics, without the EU cosmetics directive stipulating a marketing ban for cosmetical ready-to-use products to date.
General Legal Stipulations for
the Realization of Animal
Experiments in the EU
NAccording to EU Directive 86/609/EWG (EU 1986) relating to the dealing with test animals which also represents the legal basis for the German Act for the Prevention of Cruelty to Animals, according to § 7.2 "an animal experiment may not be performed if a scientifically established animal-experiment-free method is available which has been experimentally proven in practice". Further, article 23 of the same EU directive reads as follows: "EU Commission and member states are to advance research for the development and validation of alternative methods which supply the same information as the animal experiment but require a lower number of animals or are less stressing, and they are to strengthen the general conditions for this research". These legal stipulations have been implemented by several EU member states in a different manner. Germany, for example, founded already in 1989 the Zentralstelle zur Erfassung und Bewertung von Ersatz- und Ergänzungsmethoden zum Tierversuch ZEBET (Central Clearing Office for the Registration and Assessment of Replacement and Supplementary Methods for Animal Experiments) in the former Bundesgesundheitsamt (BGA) (Federal Public Health Authority), whereas most EU member states - with only few exceptions - have not initiated any own supporting programmes for alternative methods of animal experiments and rely on the activities of the EU commission.
Figure 1 shows the European centers for the furtherance of alternative
methods. The illustration explains that accordingly in Great Britain,
Nottingham, the foundation FRAME (Fund for the Replacement of Animals
in Medical Experiments) and in the Netherlands the NCA (National Center
for Alternatives) in the Reichsgesungheitsamt (RIVM) have been founded
as well as the EU Validierungszentrum ECVAM (European Center for the Validation
of Alternative Methods) in the Gemeinsame Forschungsstelle (GFS) (Common
Research Center) in ISPRA/Italy by the EU Commission in 1992. Because
of the success of the European Centers, the Validation Center ICCVAM (Interagency
Coordinating Committee for the Validation of Alternative Methods) was
founded in the frame of the National Toxicology Program NTP outside of
Europe by the US government in the year 1995 and Poland, as future joining
member of the EU established the Zentrum für Alternativmethoden "Vitryna"
(Center for Alternative Methods) in Lodsz in 2001.
|
Figure 1 |
7th Amendment of the EU
Cosmetics Directive Adopted in 2003
The 7th amendment directive 2003/15/EG (EU 2003) concerning the EU Cosmetics Directive of 1976 (EU 1976) is to lead to a ban of marketing of cosmetics in single steps, the ingredients of which have been tested in animal experiments. This gradual procedure is to entail a replacement of the majority of safety-toxicological animal tests usual so far by animal-experiment-free "Alternative Methods" within 10 years, at which the cells and tissue cultures as well as the molecular biological and molecular genetical methods are employed. In 7th amendment directive, the EU Commission has stipulated a very precise way of proceedings.
1. Already in 2003 the following animal-experiment-free
methods have to be applied:
For the test of all ready-to-use products and test of ingredients on penetration
through the skin as to caustic and phototoxic properties.
2. Until end of 2006, the following animal
tests will be given up:
Tests on eye-irritating, skin-irritating and skin-sensitizing properties.
3. Until end of 2012, i. e. in 10 years, the following
animal tests will be renounced.
Tests on embryo-toxic properties.
4. In the year 2013 the following complex
animal experiments will still be required according to the assessment
of the EU commission for the testing of cosmetics:
Tests on fertility-inhibiting and carcinogenic properties as well as the
determination of the distribution and metabolism of foreign materials
in the organism (toxico-kinetics).
In general, irrespective of this scheduling in 7th amendment directive of the EU Cosmetics Decree, all safety-toxicological alternative methods have to be applied in lieu of animal tests if they have been included as official test method for chemical substances in annex V of the EU Dangerous Substance Act (EU Directive 67/548/EEC, EU 1967) after experimental validation by the EU.
Replacement
of Safety Toxicological Safety-toxicological tests are mandatory for all chemical substances,
in fact due to reasons of industrial safety in order to protect workers
who get in contact with them in the course of the manufacturing process.
Further tests for the consumer protection are obligatory by considering
of the application, e. g. for cosmetics, food additives and drugs. Moreover,
since a few years, tests for the environmental compatibility of novel
substances have to be carried out. Internationally, it has proven successful
in toxicology to determine the dangerous properties of a substance in
a first step in internationally standardized animal tests, e. g. in Europe
according to the stipulations of the EU (EU Dangerous Substance Act, EU
Directive 67/548/EEC) and worldwide according to the test methods of the
OECD (Organization for Economic Cooperation and Development) (OECD 1982).
The toxicological test methods provided by the EU are published in annex
V of the Dangerous Substance Act. They are summarized in Table 1,
whereas the methods are particularly stressed by which the local tolerability
of the ingredients of cosmetics is tested. If the safety-toxicological animal experiments listed in Table 1
have to be substituted by animal-experiment-free methods according to
7th amendment of the EU Cosmetics directive 76/768/EEC, the new methods
have to be experimentally validated pursuant to the recommendations of
the EU and the OECD (OECD 1996) in order to prove that the results which
are obtained with the new methods allow an assessment of the dangerous
properties of new substances in the same way as the established animal
experiments. The general way of proceedings in the experimental validation
of toxicological test methods in several laboratories under blind conditions
with a sufficiently large number of test substances is schematically illustrated
in Figure 2 (OECD 1996). It is obvious that it is relatively time-consuming
and very costly till a new method has successfully undergone the long
way of validation and is also internationally recognized by all OECD member
states, i. e. by all major industrial nations. So far only 4 animal experiment
free alternative methods have been included after an approximately ten-year
experimental validation into the official test directives, namely tests
for the examination of caustic and phototoxic properties as well as penetration
through the skin and on sensitizing or allergenic effect on skin. Figure
2 The progress achieved so far concerning the development, validation and
official approval of animal-experiment free safety-toxicological test
methods are explained in the following:
1. Eye Irritation
The simple claim to substitute the severely stressing Draize-test at
the rabbit eye for the examination of eye-irritating properties by means
of one or several in-vitro methods has not been fulfilled despite large-scale
validation studies. The reason is that 1. Draize tests are
applied for various purposes, namely for tests of substances as to 2. Moreover, by means of the Draize test reactions at various parts
of the organ eye (mucous membrane, cornea and ocular lens) are assessed
and 3. urthermore, at international consideration, the reactions achieved
in the Draize-test are rated according to very differing standards by
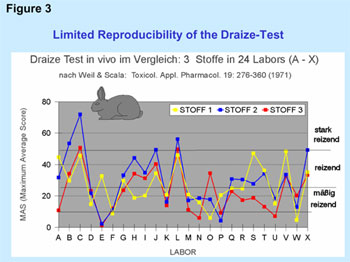
national authorities. A fundamentally well-known problem of the Draize-test at the rabbit
eye is moreover the limited reproducibility which was already proven in
a comprehensive validation study by Weil and Scala in 1971 and the result
of which is shown in Figure 3.
Animal Experiments by
Animal-test-free
Methods
Table
1:
Safety-toxicological
Test Methods of EU and OECD
Acute
tests, with nonrecurring application
•
Acute
toxicity, dermal & oral
•
Eye
irritation/ -causticity
•
Skin
irritation/-causticity
•
Skin
sensitization
•
Skin
penetration
II.
Long-term
tests with multiple applications
Sub-acute
toxicity
Sub-chronical
toxicity
>
chronical toxicity
III.
Special
and organ-specific toxicology
Metabolism
& toxico-kinetics (ADME)
Neurotoxicity
& immuno-toxicity
Teratogenicity
/ embryotoxicity
Reproduction
toxicology
•
Genotoxicity
/ mutagenicity
Carcinogenicity
•
= these tests methods are indispensable for the tolerability test
of ingredients for cosmetics.
Experimental validation of toxicological test methods: the essential
elements of the procedure for the development and experimental validation
of toxicological methods, harmonized by the OECD in 1996, which
apply in the same way to animal experiments and animal experiment-free
alternative methods (OECD 1996) are described.
- harmlessness for the application at the eye as well as
- minor and
- severely eye-irritating properties.
|
Figure
3 Draize-Test in-vivo in Comparison: 3 Substances
in 24 Laboratories (A-X). According to Weil & Scala: Toxicol.
Appl. Pharmacol. 19: 276-360 (1971) |
In Europe, a large number of alternative methods for the replacement of
the Draize-Test has been developed and validated. To date the application
of the new methods is only admitted for the classification and characterization
of substances with severely eye-irritating properties. In Figure 4
the four alternative methods are listed which have been developed and
validated in different EU member states. Test results, which have been
obtained with the four in-vitro tests, are approved by all EU member states.
Less positive is the situation outside of Europe, although the OECD calls
with emphasis for the development of alternative methods for the Draize-test
at the rabbit eye by its member states. Animal-experiment free in-vitro
tests for a differentiation of non- and minor eye-irritating properties
which are of importance for the cosmetics industry, have not yet been
accepted for official purposes despite intensive experimental validation.
The 7th amendment of the EU Cosmetics Directive takes that into consideration.
Figure
4
2. Irritating Effect on the Skin
The results of the Draize-test at the rabbit skin for testing of skin-irritating
properties are - according to expert opinions of the DG Gesundheit und
Verbraucherschutz (Health and Consumer Protection) - only partly transferable
to humans. ECVAM and the OECD are in favor of a new test strategy in which
in-vitro methods, which have been proven in toxicological laboratories
of the industry, are combined with a subsequent tolerability test at human
voluntary testees. Several EU and OECD member states, such as for instance
Germany and Austria decline in principle the test at humans instead of
animals test due to ethical reasons. An ECVAM validation study with artificial
human skin (Model EpiDerm ), performed from 1999 to 2000, has unfortunately
not yet yielded the successful result, which had been expected, due to
the insufficient reproducibility of the biological damaging parameters.
In the year 2003 the ECVAM has started jointly with the US validation
center ICCVAM an international validation study with commercial human
skin models. It will depend on the outcome of the study, in which all
major producers of cosmetics participate whether or not tests of the examination
of skin irritating properties can be renounced in the end of 2006, without
endangering the consumer protection, as designated in 7th amendment of
the EU Cosmetics Directive.
3. Causticity on Skin and Mucous Membranes
In 1998 ECVAM has successfully terminated a validation study with three
in-vitro methods for the testing of causticity on the skin, namely with
rat skin and artificial human skin (Episkin & EpiDerm ). This concerns
biotechnologically produced, commercially marketed human skin models.
In the year 2000, these validated methods were entered as official test
methods in annex V of the Dangerous Substance Act (EU Directive 67/548/EEC)
(EU Commission, 2000a) by the EU commission. Therefore, they have to be
applied under EU directive 86/609/EWG for the protection of test animals
and in the EU member states animal experiments for this purpose are prohibited.
However, particularly encouraging is the fact that in 2002 the expert
commission of the OECD accepted a special method to test the causticity
with human skin models for the worldwide application (OECD 2002a). The
successful development and validation of this in-vitro method with the
skin model EpiDerm has been coordinated by the German validation center
ZEBET. 4. Skin Sensitization
Sensitizing or allergenic effects are the most frequent unwanted property
of chemical substances (10 percent). In the case of substances, which
are to be applied in cosmetics, sensitizing properties are not acceptable.
Therefore, a test on sensitizing properties in the safety-toxicological
test of cosmetics is indispensable. The sensitization test in the skin
of guinea pigs according to Magnusson and Kligman, usual so far, is severely
stressing because adjuvants for the reaction enhancing are injected in
the skin and the immunological reaction of the damaged guinea pig skin
is tested. Since 2001 the EU member states, the USA and all OECD member
states accept the considerably less stressing LLNA (local lymph node assay),
which is performed at the ear-lymph nodes of mice, the growth inhibition
of which is tested in in-vitro culture (OECD 2002b). The LLNA is in fact
no pure in-vitro test but rather an "ex-vivo test", at which
the stress for the test animal is considerably reduced.
5. Penetration Through the Skin
For substances, which are to be applied in cosmetics or pesticides, the
absorption through the skin is decisive for the effect in the entire body
as to the safety-toxicological assessment. The EU expert commission for
cosmetics, the SCCNFP, has fundamentally decided that ingredients of cosmetics
do not have to be toxicologically tested on systemic effects if they are
not absorbed by the skin. Therefore, the test of the skin penetration
is of major importance for the tolerability test of cosmetics. In June
2001, the OECD recommended the test of skin penetration with human skin,
which originates from operation material as one of the first worldwide
valid in-vitro toxicity test for approval (OECD 2002c).
5. Photo-toxicity
Since 1992, ZEBET has coordinated a EU/COLIPA pre-validation and validation
study of in-vitro photo-toxicity tests that was successfully terminated
in 1998 with the experimental validation of the 3T3 NRU in-vitro photo-toxicity
tests (3T3 NRU PT test) at which a fibroblast cell line of mice is applied.
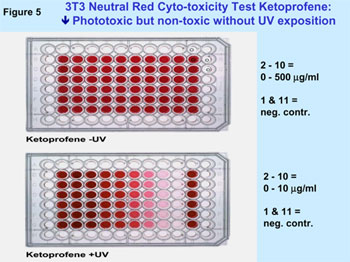
Figure 5 illustrates a typical example for a phototoxic substance
in 3T3 NRU in the in vitro photo-toxicity test. The results of the validation
study with 30 test substances under blind conditions in 10 laboratories
in Europe and the USA as well as an additional validation study with 10
UV-filter substances which are applied in sun protection products, confirmed
that the results in the 3T3 test allow a better prediction of the photo-toxic
properties for humans than with every other in-vivo or in-vitro test method
(Spielmann et al., 1998 a,b). Therefore, in 2000 the EU commission included
the 3T3 NRU PT test as single photo-toxicity test in annex V of the Dangerous
Substance Act (EU Commission, 2001c) so that only this in-vitro test can
be applied for official purposes in Europe and moreover that animal experiments
for this purpose are prohibited. Further, in the year 2002, an expert
commission of the OECD has accepted the 3T3 NRU PT as official worldwide
test method for the photo-toxicity test (OECD, 2002d). Thus, the 3T3 NRU
PT test is the first in-vitro-toxicity test, which is accepted worldwide
after successful experimental validation for official purposes. Figure
5
Animal Protection and The scientific advisory board of the EU validation center ECVAM assessed
in 2001, in connection with the proposal of the EU Commission for a new
chemicals policy that in approximately five years there will be animal
experiment-free-alternative methods for toxicological test methods available.
The assessment summarized in Figure 6 clarifies that for the most
important fields in toxicology there will be animal-experiment-free methods
available (EU Commission 2001) very soon. Figure
6
References
EU Kommission (1976) EU Richtlinie 76/768/EEC zur Angleichung
der gesetzlichen Grundlagen für kosmetische Produkte in den Mitgliedsstaaten.
Brüssel, Belgien: EU DG Umwelt. EU Kommission (1986)
EU Richtlinie 86/609/EEC zu Schutz von Versuchstieren. Brüssel, Belgien: EU DG Umwelt. EU Kommission (1983)
EU Richtlinie 83/467EEC zur fünften technischen Anpassung der EU Richtlinie
67/548/EEC an die Gesetzgebung für die Klassifizierung, Verpackung und
Kennzeichnung von Gefahrstoffen. Brüssel, Belgien: EU DG Umwelt. EU Kommission (2000a)
EU Richtlinie 2000/33/EU für die 21st Änderung von Anhang V
der EU Richtinie 67/548/EEC zur Klassifizierung, Verpackung und Kennzeichnung
von Gefahrstoffen: Test Methode B-40 "Hautätzung - in vitro Methode”.
O. J. der EU Kommission vom 8. Juni 2000, L136, 85-97. EU Kommission (2000b)
EU Richtlinie 2000/33/EU für die 21st Änderung von Anhang V
der EU Richtinie 67/548/EEC zur Klassifizierung, Verpackung und Kennzeichnung
von Gefahrstoffen: Test Methode B-41 "Phototoxizität – in vitro 3T3
NRU Phototoxizitätstest ”. O. J. der EU Kommission vom 8. Juni 2000, L136,
85-97. EU Kommission (2001).
Weissbuch für eine neue Chemikalienpolitik. Brüssel, Belgien im Internet:
EU Kommission (2003)
EU Richtlinie 2003/15/EG zur Änderung der Richtlinie 76/768/EWG des rates
zur Angleichung des rechtsvorschriften der Mitgliedsstaaten über kosmetische
Mittel. O. J. der EU Kommission vom 11. März 2003, L66,
26-35 OECD (Organisation for Economic Co-operation and Development) (1982)
OECD Guidelines for Testing of Chemicals. Paris, Frankreich: OECD Publikation
Office. OECD (1996) Final Report of the OECD Workshop on Harmonization of Validation
and Acceptance Criteria for Alternative Toxicological Tests Methods. Paris,
Frankreich: OECD Publication Office. OECD (2002a) OECD guidelines for the testing of chemicals: Test Guideline
431 "In vitro skin corrosion: human skin model ". Paris, Frankreich, OECD Publication Office. OECD (2002b) OECD guidelines for the testing of chemicals: Test Guideline
429 "Skin sensitisation: Local Lymph Node Assay. Paris, Frankreich, OECD Publication Office OECD (2002c) OECD guidelines for the testing of chemicals: Test Guideline
428 "Skin absorption: in vitro method ". Paris, Frankreich, OECD Publication Office. OECD (2002d) OECD guidelines for the testing of chemicals: Test Guideline
432 "In vitro 3T3 NRU phototoxicxity test". Paris, Frankreich, OECD Publication Office. Spielmann, H., Balls, M., Dupuis, J., Pape, W.J.W., Pechovitch G., de
Silva, O., Holzhütter, H.G., Clothier, R., Desolle, P., Gerberick, G.F.,
Liebsch, M., Lovell, W.W., Maurer, T., Pfannenbecker, U., Potthast, J.M.,
Csato, M., Sladowski, D., Steiling, W. und Brantom, P.: The international
EU/COLIPA in vitro phototoxicity validation study: results of phase II
(blind trial), part 1: the 3T3 NRU Phototoxicity test. Toxic. in Vitro
12, 305-327, 1998a Spielmann, H., Balls, M. Dupuis, J., Pape, W.J.W., de Silva, O., Holzhütter,
H.G., Gerberick, F., Liebsch, M., Lovell, W.W., Pfannenbecker, U. und
Brantom, P.: A special study on the phototoxic potential of UV filter
chemicals from Annex II of the REU Directive 76/86 in the 3T3 NRU in vitro
phototoxicity test. ATLA 26, 979-708, 1998b Weil, C.S. und Scala, R.A.: Study of intra- and interlaboratory variability
in the results of rabbit eye and skin irrtiation tests. Toxicol. Appl. Pharmacol. 19, 276-360; 1971
Alternative methods for tests of substances with severely eye-irritating
properties, which have been approved for official tests in the
EU. It concerns the HET-CAM test at the incubated hen's egg, the
BCOP (bovine cornea opacity and permeability) test, the IRE (isolated
rabbit eye) test at the rabbit eye and the ICE (isolated chicken
eye) test at the chicken eye. The eyes, respectively, are from
slaughterhouse material.
Example for a phototoxic substance (Ketoprofene), which entails
a positive result in the 3T3 NRU PT in-vitro photo-toxicity test.
The test is performed in a micro-titer plate with 96 reaction
receptacles. UV radiation leads to a phototoxic reaction with
the phototoxic substance in the lower plate that entails relation-dependent
to decoloring.
Development of Novel Cosmetics are Compatible
Special fields of toxicology in which, according to the opinion
of the scientific advisory board of the EU validation center ECVAM,
validated animal-experiment-free toxicological test methods will
be available in the coming 5 years.
The results presented explain that the EU legislation in the sector cosmetics
for which a particularly high priority is given to the EU Directive 86/609/EWG
for the protection of test animals does not lead to a standstill of research
and new development, as is often maintained, but the cosmetics industry
in Europe also takes the leading position scientifically in the field of
toxicological safety tests in comparison with Japan and the USA. The EU
Animal Protection Act has therefore lead to the market leadership in the
world market in the cosmetics sector. The 7th amendment of the Cosmetics
Directive of the EU is an important milestone for the strengthening of the
position of the European cosmetics industry in the world market. It is,
furthermore, conceived following the reality and takes primarily the consumer
requirements into consideration without neglecting the scientific progress
and animal protection. The example of the cosmetics industry explains that
innovative economical developments connected with a leading position in
the world market and topical animal protection is not mutually exclusive
but complements one another.
Test animals do not have to suffer any longer today from safety
toxicological tests of cosmetics.
http://europa.eu.int/comm/environment/chemicals/0188_de.pdf
Author
Professor Dr. med. Horst Spielmann

Zentralstelle zur Erfassung und Bewertung von Ersatz- und Ergänzungsmethoden
zum Tierversuch (ZEBET) (Central Association for the Registration and Assessment
of Substitution- and Supplementary Methods for Animal Experiments) at the
Bundesinstitut für Risikobewertung (BfR) (Federal Institute for Risk
Assessment) in Berlin
Address:
Dr. med. Horst Spielmann
Direktor und Professor
Leiter ZEBET im BfR
Diedersdorfer Weg 1
12277 BERLIN
Tel: 01888-412-2270
Fax: 01888-412-2958
E-mail: spielmann.zebet@bfr.bund.de
top